Oxidized
--------------------------------------------------------------
You Are Here -> Home ->Oxidized
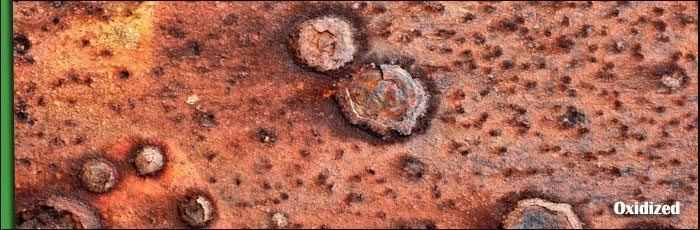
What is Oxidized?
Oxidation is a chemical process by which an ionic chemical reaction occurs at the surface of a metal when in the presence of oxygen. This can happen in air or when metal is exposed to water or acids. The most common example is the rusting of steel, which is a transformation of the iron molecules on the surface of the steel into iron oxides, most commonly Fe2O3 and Fe3O4. Noble metals are those which strongly resist oxidation in their natural state, such as platinum or gold, while many corrosion-resistant alloys have been invented by man, such as stainless steels and bras
The Process of Oxidation
When an atom or compound is oxidized its properties change. For example, when an iron object undergoes oxidation it is transformed because it has lost electrons. Unoxidized iron is a strong, structurally sound metal, while oxidized iron is a brittle reddish powder. The diagram below illustrates what happens to an atom of iron as it is oxidized.
Once iron has been oxidized, it carries a charge. Because it lost three electrons, it now has a positive charge of three. This is positive three charge is represented by the number three and a positive sign (3+) written as a superscript to the right of the Iron (Fe) symbol.
Iron is very easily oxidized, which is why its important to minimize the exposure of iron to oxygen and moisture. Iron will continue to lose electrons to oxygen as long as oxygen is present.
Oxidation-Reduction (Redox) Reactions
Most of the time, oxidation occurs in tandem with a process called reduction. Reduction is the process of gaining one or more electrons. In an oxidation-reduction or redox reaction, one atom or compound will steal electrons from another atom or compound.
A classic example of a redox reaction is rusting. When rusting happens, oxygen steals electrons from iron. Oxygen gets reduced while iron gets oxidized. The result is a compound called iron oxide, or rust. Unoxidized or pure iron is distinctly different from the oxidized form that occurs in rust.
------------------------------------------------------------------------------------------------------------------------------
For More Information About Oxidized please call +91 22 2385 6385 / 2385 638 or send an email to info@jfengg.com
